The American Diabetes Association (ADA) “Standards of Care in Diabetes” includes the ADA’s current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, an interprofessional expert committee, are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA’s clinical practice recommendations and a full list of Professional Practice Committee members, please refer to Introduction and Methodology. Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC.
Recommendations
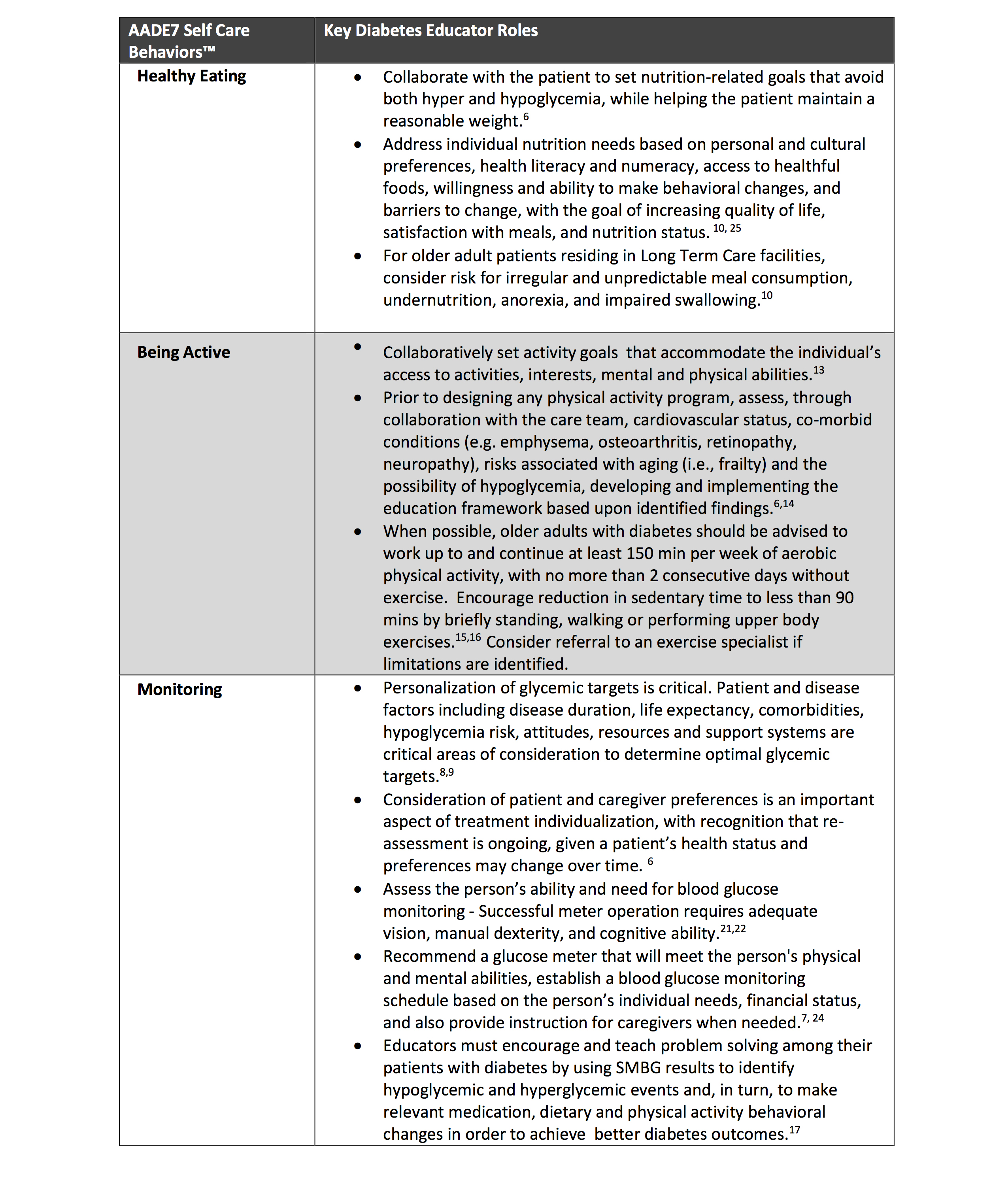
• 13.1 Consider the assessment of medical, psychological, functional (self-management abilities), and social domains in older adults with diabetes to provide a framework to determine goals and therapeutic approaches for diabetes management. B
• 13.2 Screen for geriatric syndromes (e.g., cognitive impairment, depression, urinary incontinence, falls, persistent pain, and frailty) and polypharmacy in older adults with diabetes, as they may affect diabetes self-management and diminish quality of life. B
Diabetes is a highly prevalent health condition in the aging population. Over one-quarter of people over the age of 65 years have diabetes and one-half of older adults have prediabetes (1,2). The number of older adults living with these conditions is expected to increase rapidly in the coming decades. Diabetes in older adults is a highly heterogeneous condition. While type 2 diabetes predominates in the older population as in the younger population, improvements in insulin delivery, technology, and care over the last few decades have led to increasing numbers of people with childhood and adult-onset type 1 diabetes surviving and thriving into their later decades. Diabetes management in older adults requires regular assessment of medical, psychological, functional, and social domains. When assessing older adults with diabetes, it is important to accurately categorize the type of diabetes as well as other factors, including diabetes duration, the presence of complications, and treatment-related concerns, such as fear of hypoglycemia. Screening for diabetes complications in older adults should be individualized and periodically revisited, as the results of screening tests may impact treatment goals and therapeutic approaches (3–5). Older adults with diabetes have higher rates of functional disability, accelerated muscle loss, and coexisting illnesses, such as hypertension, chronic kidney disease, coronary heart disease, and stroke, and of premature death than those without diabetes. At the same time, older adults with diabetes also require greater caregiver support and are at greater risk than other older adults for several common geriatric syndromes such as cognitive impairment, depression, urinary incontinence, injurious falls, persistent pain, and frailty as well as polypharmacy (1). These conditions may impact older adults’ diabetes self-management abilities and quality of life if left unaddressed (2,6,7). See Section 4, “Comprehensive Medical Evaluation and Assessment of Comorbidities,” for the full range of issues to consider when caring for older adults with diabetes.
The comprehensive assessment described above provides a framework to determine goals and therapeutic approaches (8–10), including whether referral for diabetes self-management education is appropriate (when complicating factors arise or when transitions in care occur) or whether the current plan is too complex for the individual’s self-management ability or the caregivers providing care (11). Particular attention should be paid to complications that can develop over short periods of time and/or would significantly impair functional status, such as visual and lower-extremity complications. Please refer to the American Diabetes Association (ADA) consensus report “Diabetes in Older Adults” for details (3).
Neurocognitive Function
Recommendation
• 13.3 Screening for early detection of mild cognitive impairment or dementia should be performed for adults 65 years of age or older at the initial visit, annually, and as appropriate. B
Older adults with diabetes are at higher risk of cognitive decline and institutionalization (12,13). The presentation of cognitive impairment ranges from subtle executive dysfunction to memory loss and overt dementia. People with diabetes have higher incidences of all-cause dementia, Alzheimer disease, and vascular dementia than people with normal glucose tolerance (14). Poor glycemic management is associated with a decline in cognitive function (15,16), and longer duration of diabetes is associated with worsening cognitive function. There are ongoing studies evaluating whether lifestyle interventions may help to maintain cognitive function in older adults (17). However, studies examining the effects of diabetes prevention or intensive glycemic and blood pressure management to achieve specific goals have not demonstrated a reduction in brain function decline (18,19). In observational studies as well as post hoc analyses from randomized clinical trials, certain glucose-lowering drugs, such as metformin, thiazolidinediones, and glucagon-like peptide 1 (GLP-1) receptor agonists have shown small benefits on slowing progression of cognitive dysfunction (20). Cardiovascular risk factors are also associated with an increased risk of cognitive decline and dementia. Control of blood pressure and cholesterol lowering with statins have been associated with a reduced risk of incident dementia and are, thus, particularly important in older adults with diabetes.
Recently, the U.S. Food and Drug Administration (FDA) has approved two new anti-amyloid monoclonal antibodies for the treatment of early Alzheimer disease (21). These drugs lower the amyloid burden in the brain and appear to slow cognitive decline in the populations tested. Whether these drugs will be useful in other populations including older adults with diabetes remains to be determined.
Despite the paucity of therapies to prevent or remedy cognitive decline, identifying cognitive impairment early has important implications for diabetes care. The presence of cognitive impairment can make it challenging for clinicians to help people with diabetes reach individualized glycemic, blood pressure, and lipid goals. Cognitive dysfunction may make it difficult for individuals to perform complex self-care tasks (22), such as monitoring glucose and adjusting insulin doses. It can also hinder their ability to appropriately maintain the timing of meals and content of the diet. These factors increase risk for hypoglycemia, which, in turn, can worsen cognitive function. When clinicians are providing care for people with cognitive dysfunction, it is critical to simplify care plans and to facilitate and engage the appropriate support structure to assist individuals in all aspects of care.
Older adults with diabetes should be carefully screened and monitored for cognitive impairment (2). Several simple assessment tools are available to screen for cognitive impairment (22,23), such as the Mini-Mental State Examination (24), Mini-Cog (25), and the Montreal Cognitive Assessment (26), which may help to identify individuals requiring neuropsychological evaluation, particularly when dementia is suspected (i.e., in those experiencing memory loss, a decrease in executive function, and declines in their basic and instrumental activities of daily living). Annual screening is indicated for adults 65 years of age or older for early detection of mild cognitive impairment or dementia (4,27). Screening for cognitive impairment should additionally be considered when an individual presents with a significant decline in clinical status due to increased problems with self-care activities and medication management, such as errors in calculating insulin dose, difficulty counting carbohydrates, skipped meals, skipped insulin doses, and difficulty recognizing, preventing, or treating hypoglycemia. People who screen positive for cognitive impairment should receive diagnostic assessment as appropriate, including referral to a behavioral health professional for formal cognitive/neuropsychological evaluation (28).
Hypoglycemia
Recommendations
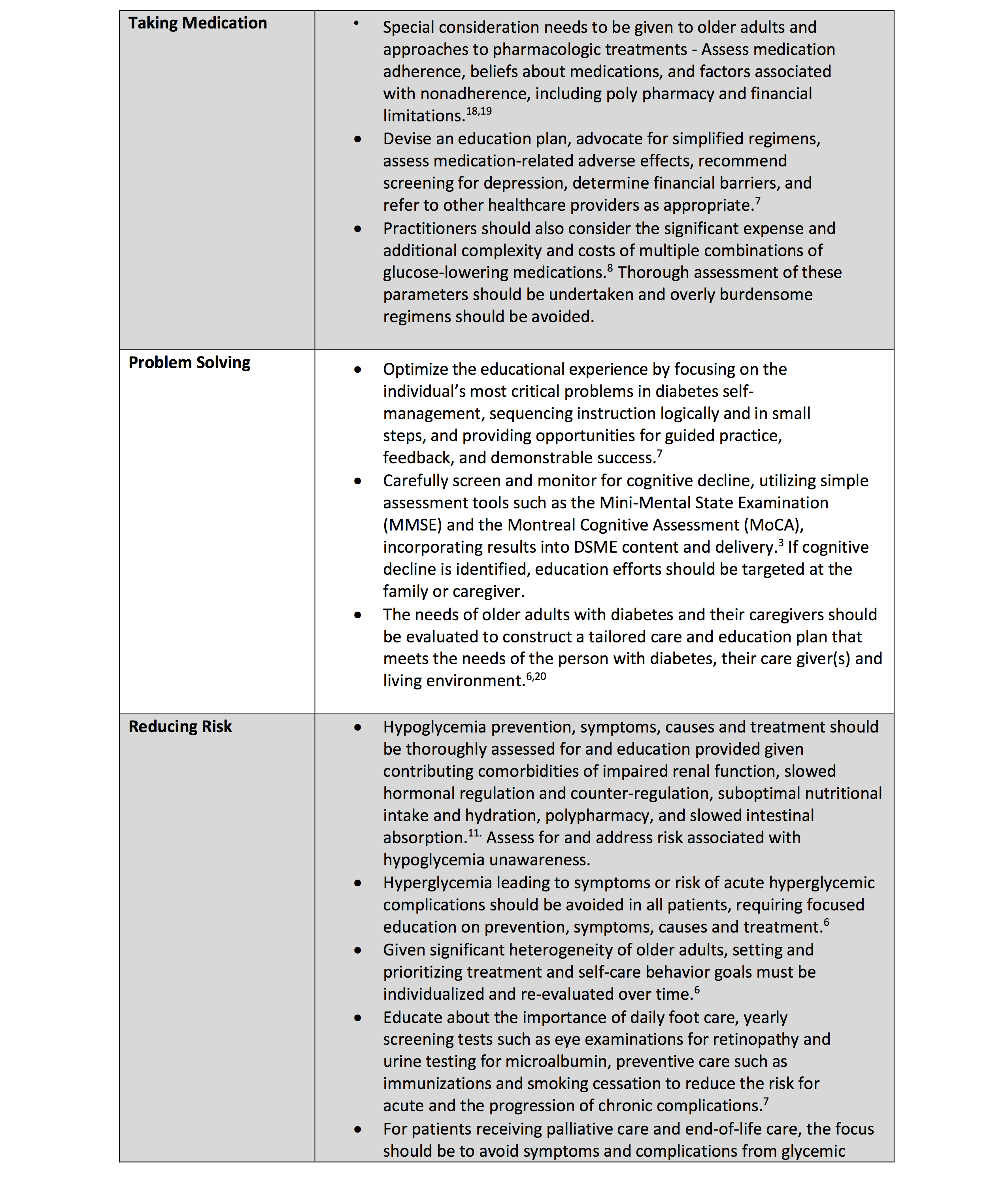
• 13.4 Because older adults with diabetes have a greater risk of hypoglycemia, especially when treated with hypoglycemic agents (e.g., sulfonylureas, meglitinides, and insulin), than younger adults, episodes of hypoglycemia should be ascertained and addressed at routine visits. B
• 13.5 For older adults with type 1 diabetes, continuous glucose monitoring is recommended to reduce hypoglycemia. A
• 13.6 For older adults with type 2 diabetes on insulin therapy, continuous glucose monitoring should be considered to improve glycemic outcomes and reduce hypoglycemia. B
• 13.7 For older adults with type 1 diabetes, consider the use of automated insulin delivery (AID) systems A and other advanced insulin delivery devices such as connected pens E to reduce risk of hypoglycemia, based on individual ability and support system.
Older adults are at higher risk of hypoglycemia for many reasons, including erratic meal intake, insulin deficiency necessitating insulin therapy, and progressive renal insufficiency (29). As described above, older adults have higher rates of unidentified cognitive impairment and dementia, leading to difficulties in adhering to complex self-care activities (e.g., glucose monitoring and insulin dose adjustment). Cognitive decline has been associated with increased risk of hypoglycemia, and conversely, severe hypoglycemia has been linked to increased risk of dementia (30–32). Therefore, as discussed in Recommendation 13.3, it is important to routinely screen older adults for cognitive impairment and dementia and discuss findings with the individuals and their caregivers.
People with diabetes and their caregivers should be routinely queried about hypoglycemia (e.g., selected questions from the Diabetes Care Profile) (33) and impaired hypoglycemia awareness as discussed in Section 6, “Glycemic Goals and Hypoglycemia.” Older adults can also be stratified for future risk for hypoglycemia with validated risk calculators (e.g., Kaiser Hypoglycemia Model) (34) and with consideration of hypoglycemia risk factors (Table 6.5). An important step to mitigate hypoglycemia risk is to determine whether the person with diabetes is skipping meals or inadvertently repeating doses of their medications. Glycemic goals and pharmacologic treatments may need to be adjusted to minimize the occurrence of hypoglycemic events (2). This recommendation is supported by results from multiple randomized controlled trials, such as the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study and the Veterans Affairs Diabetes Trial (VADT), which showed that intensive treatment protocols aimed to achieve an A1C <6.0% with complex drug plans significantly increased the risk for hypoglycemia requiring assistance compared with standard treatment (35,36). However, these intensive treatment plans included extensive use of insulin and minimal use of GLP-1 receptor agonists, and they preceded the availability of sodium–glucose cotransporter 2 (SGLT2) inhibitors.
Use of Continuous Glucose Monitoring and Advanced Insulin Delivery Devices
For older adults with type 1 diabetes, continuous glucose monitoring (CGM) is a useful approach to predicting and reducing the risk of hypoglycemia (37). In the Wireless Innovation in Seniors with Diabetes Mellitus (WISDM) trial, adults over 60 years of age with type 1 diabetes were randomized to CGM or standard blood glucose monitoring. Over 6 months, use of CGM resulted in a small but statistically significant reduction in time spent with hypoglycemia (glucose level <70 mg/dL) compared with standard blood glucose monitoring (adjusted treatment difference −1.9% [−27 min/day]; 95% CI −2.8% to −1.1% [−40 to −16 min/day]; P < 0.001) (38,39). Among secondary outcomes, time spent in range between 70 and 180 mg/dL increased by 8% (95% CI 6.0–11.5) and glycemic variability (%CV) decreased. A 6-month extension of the trial demonstrated that these benefits were sustained for up to a year (40). These and other short-term trials are supported by observational data from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study indicating that among older adults (mean age 58 years) with long-standing type 1 diabetes, routine CGM and insulin pump use was associated with fewer hypoglycemic events and hyperglycemic excursions and lower A1C levels (41). While the current evidence base for older adults is primarily in type 1 diabetes, the evidence demonstrating the clinical benefits of CGM for people with type 2 diabetes using insulin is growing (42) (see Section 7, “Diabetes Technology”). The DIAMOND (Multiple Daily Injections and Continuous Glucose Monitoring in Diabetes) study demonstrated that in adults ≥60 years of age with either type 1 or type 2 diabetes using multiple daily injections, CGM use was associated with improved A1C and reduced glycemic variability (43). Older adults with physical or cognitive limitations who require monitoring of blood glucose by a surrogate or reside in group homes or assisted living centers are other populations for which CGM may play a useful role.
The availability of accurate CGM devices that can communicate with insulin pumps through Bluetooth has enabled the development of advanced insulin delivery algorithms for pumps. These algorithms fall into two categories: predictive low-glucose suspend algorithms that automatically shut off insulin delivery if a hypoglycemic event is imminent and hybrid closed-loop algorithms that automatically adjust insulin infusion rates based on feedback from a CGM to keep glucose levels in a goal range. Advanced insulin delivery devices have been shown to improve glycemic outcomes in both children and adults with type 1 diabetes. Most trials of these devices have included a broad range of people with type 1 diabetes but relatively few older adults. Recently, two small randomized controlled trials in older adults have been published. The Older Adult Closed Loop (ORACL) trial in 30 older adults (mean age 67 years) with type 1 diabetes found that an automated insulin delivery (AID) strategy was associated with significant improvements in time in range compared with sensor-augmented pump therapy (44). Moreover, they found small but significant decreases in hypoglycemia with the AID strategy. Boughton et al. (45) reported results of an open-label, crossover design clinical trial in 37 older adults (≥60 years) in which 16 weeks of treatment with a hybrid closed-loop advanced insulin delivery system was compared with sensor-augmented pump therapy. They found that hybrid closed-loop insulin delivery improved the proportion of time glucose was in range largely due to decreases in hyperglycemia. In contrast to the ORACL study, no significant differences in hypoglycemia were observed. Both studies enrolled older individuals whose blood glucose was relatively well managed (mean A1C ∼7.4%), and both used a crossover design comparing hybrid closed-loop insulin delivery to sensor-augmented pump therapy. These trials provide the first evidence that older individuals with long-standing type 1 diabetes can successfully use advanced insulin delivery technologies to improve glycemic outcomes, as has been seen in younger populations. A recent real world evidence analysis of a Medicare population (n = 4,243, 89% with type 1 diabetes, mean age 67.4 years) also indicated that initiating hybrid closed-loop insulin delivery was associated with improvements in mean glucose and a 10% increase in time in range (46). Use of such technologies should be periodically reassessed, as the burden may outweigh the benefits in those with declining cognitive or functional status.
Treatment Goals
Recommendations
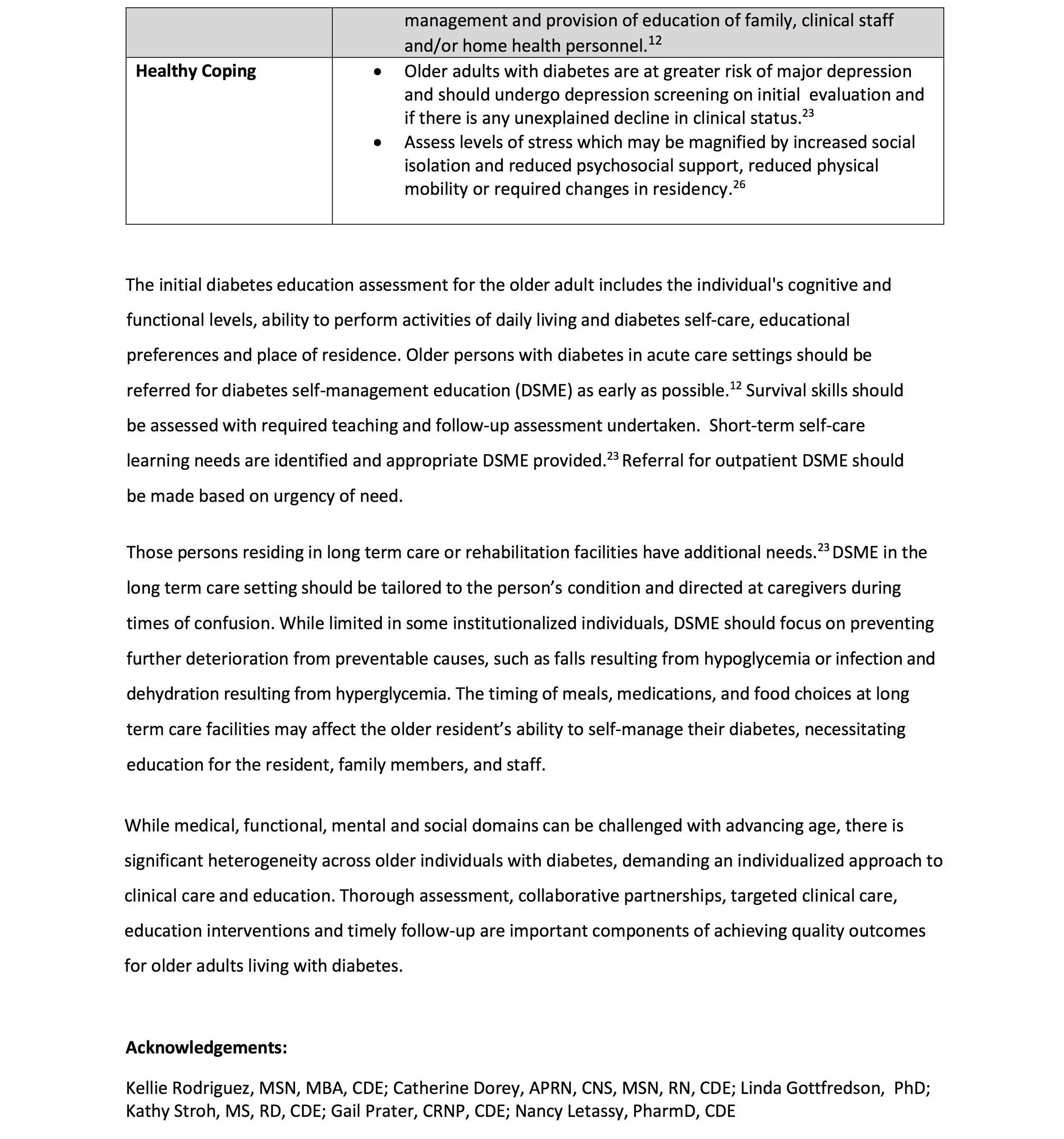
• 13.8a Older adults with diabetes who are otherwise healthy with few and stable coexisting chronic illnesses and intact cognitive function and functional status should have lower glycemic goals (such as A1C <7.0–7.5% [<53–58 mmol/mol]).
• 13.8b Older adults with diabetes and intermediate or complex health are clinically heterogeneous with variable life expectancy. Selection of glycemic goals should be individualized, with less stringent goals (such as A1C <8.0% [<64 mmol/mol]) for those with significant cognitive and/or functional limitations, frailty, severe comorbidities, and a less favorable risk-to-benefit ratio of diabetes medications.
• 13.8c Older adults with very complex or poor health receive minimal benefit from stringent glycemic control, and clinicians should avoid reliance on glycemic goals and instead focus on avoiding hypoglycemia and symptomatic hyperglycemia.
• 13.9 Screening for diabetes complications should be individualized in older adults with diabetes. Particular attention should be paid to complications that would lead to impairment of functional status or quality of life.
• 13.10 Treatment of hypertension to individualized goal levels is indicated in most older adults with diabetes.
• 13.11 Treatment of other cardiovascular risk factors should be individualized in older adults with diabetes, considering the time frame of benefit. Lipid-lowering therapy and antiplatelet agents may benefit those with life expectancies at least equal to the time frame of primary prevention or secondary intervention trials.
The care of older adults with diabetes is complicated by their clinical, cognitive, and functional heterogeneity and their varied prior experience with disease management. Some older individuals may have developed diabetes years earlier and have significant complications, others are newly diagnosed and may have had years of undiagnosed diabetes with resultant complications, and still, other older adults may have truly recent-onset disease with few or no complications (47). Some older adults with diabetes have other underlying chronic conditions, substantial diabetes-related comorbidity, limited cognitive or physical functioning, or frailty (48,49). Other older individuals with diabetes have little comorbidity and are active.
Life expectancies are highly variable but are often longer than clinicians realize. Multiple prognostic tools for life expectancy for older adults are available (50,51). Notably, the Life Expectancy Estimator for Older Adults with Diabetes (LEAD) tool was developed and validated among older adults with diabetes, and a high risk score was strongly associated with having a life expectancy of <5 years (52). These data may be a useful starting point to inform decisions about selecting less stringent glycemic goals (52,53). Older adults also vary in their preferences for the intensity and mode of glucose management (54). Health care professionals caring for older adults with diabetes must take this heterogeneity into consideration when setting and prioritizing treatment goals (9,10) (Table 13.1 ). In addition, older adults with diabetes should be assessed for disease treatment and self-management knowledge, health literacy, and mathematical literacy (numeracy) at the onset of treatment. See Fig. 6.2 for individual/disease-related factors to consider when determining individualized glycemic goals.
to read full article click here: https://diabetesjournals.org/care/article/47/Supplement_1/S244/153944/13-Older-Adults-Standards-of-Care-in-Diabetes-2024

You must be logged in to post a comment.